Testing and Counting Method of Coliform Bacteria
Published date and time: 2020-9-29, 08:32
Date and time of modification: 2021-05-07, 19:50
Author: Food-Men
This article introduces the methods for the testing and counting of coliform bacteria in food. The method of MPN counting is suitable for counting with lower coliform content, and the method of plate media count is suitable for counting with higher content.
Principles of Testing and Counting Method of Coliform Bacteria
Coliforms bacteria are commonly found in the feces of warm-blooded animals, including humans. By testing and counting coliform bacteria, you can determine the degree of food contamination with bacteria. Individual coliform is generally difficult to see with the naked eye, but after it is reared, it grows from a single bacterium to a flora, which can be easily seen and counted. The total number of coliform bacteria can show the sanitation status of foods in general. Therefore, many countries have strict regulations on the total number of coliform bacteria in foods. In addition, coliform bacteria can ferment lactose and generate acids and gases when incubated at 35–37 ° C. Using this feature can analyze the number of coliform bacteria in food. There are two ways to count the coliforms here:
1. MPN Counting Method for Coliform
1.1 Operation Steps for MPN Counting of Coliforms
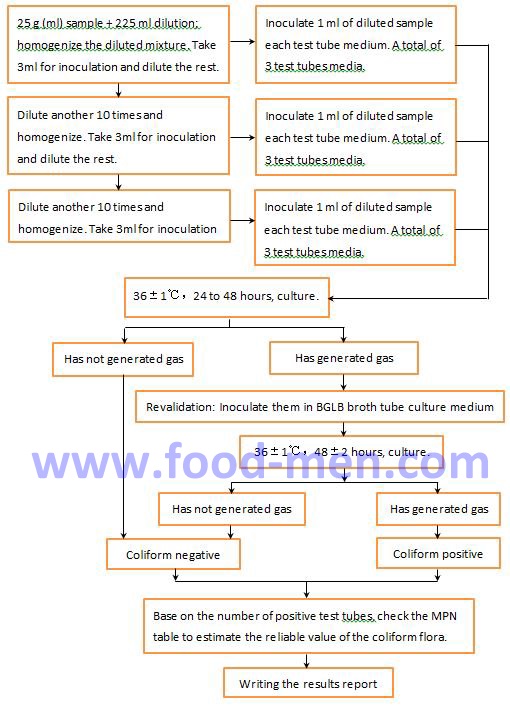 1.2 Instruction of Operation for MPN Counting of Coliforms
1.2 Instruction of Operation for MPN Counting of Coliforms
If bacteria are present on the medium but no gas is generated, the coliform flora is negative. However, further analysis is needed to determine whether the types of these bacteria are harmful to the human body. Find out what caused these bacteria.
2. Plate Media Counting Method for Coliform
2.1 Operation Steps for Plate Counting of Coliform
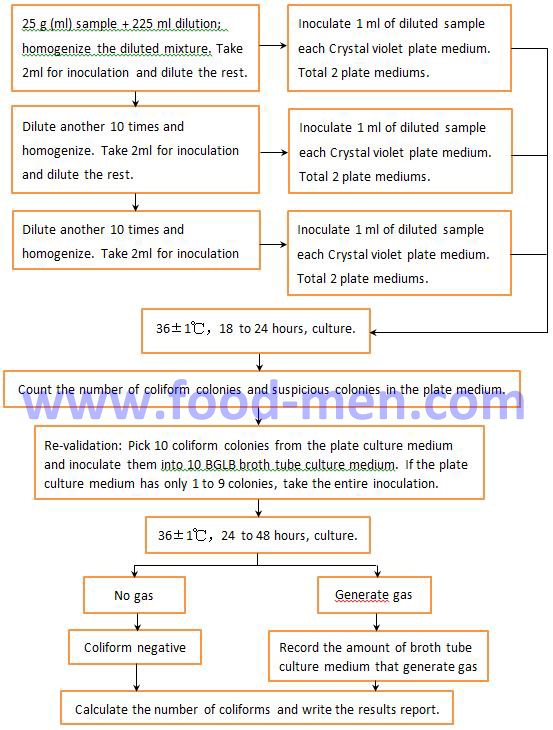 2.2 Instruction of Operation for Plate Counting of Coliform
2.2 Instruction of Operation for Plate Counting of Coliform
• If there are colonies on the plate medium, but later re-verification does not generate gas. Although it is negative for the coliform flora, further analysis and testing are needed to determine whether the type of bacteria is harmful to the human body.
• Calculation of coliform bacteria: N = A × (C / B) × T
Among them:
N ---- The number of coliforms bacteria
A ---- The number of colonies on the plate medium. Count with an automatic colony counter or manually.
B ---- The number that pick the colonies from the plate culture medium and inoculate into BGLB broth test tube medium, when re-verifying. Generally 10 or less. Use an inoculation loop or inoculation needle to pick colonies and inoculate.
C ---- The number of BGLB broth test tube culture medium that generate gas during re-verification
T ---- Multiples of sample dilution
3. Cautions for Operation of Coliform Counting
(Click on the blue font to browse another page to view the relevant introduction)
3.1 All tools that contact the contents of the sample should be sterilized by Horizontal Laboratory Autoclave Sterilizers or Vertical Laboratory Autoclave Sterilizers.
3.2 The operation of the sample opening or after opening the lid shall be carried out in an air-cleaned environment, that is aseptic operation. The aseptic operation can be performed in a sterile laboratory (clean room) or clean benches.
3.3 If the contents are a mixture of solid and liquid, put them in Sterile Sample Blender Bags, seal the bags, pat with stomacher blenders, break the solids of the contents, and then inspect.
3.4 If the contents are multiple liquids and you want to stir evenly, use a Magnetic Stirrer Mixer with Timer and HotPlate or a 4 Heads Magnetic Stirrer Mixer with HotPlate or Multifunctional Orbital Shaker Incubators for Laboratory, to ensure that there are no contaminated by microorganisms when are stirring.
3.5 A blank test is required (sample test of no microorganisms). When there is a microbe in the blank test, it indicates that there is a problem with the test operation and the result is not credible.
4. Preparation of culture medium for coliform test
4.1 Lauryl sulfate tryptose (LST) broth
4.1.1 Composition
Typtone or Trypticase: 20.0g
Sodium chloride: 5.0g
Lactose: 5.0g
K2HPO4: 2.75g
KH2PO4: 2.75g
Lauryl sodium sulfate: 0.1g
Distilled water: 1000mL
4.1.2 Preparation method
Dissolve all the above components in distilled water and adjust the pH to 6.8±0.2. Dispense 10mL of the solution into each test tube which possesses small glass backward tubes, and sterilize for 15min in the autoclave set at 121℃.
4.2 Brilliant green lactose bile (BGLB) broth
4.2.1 Composition
Peptone: 10.0g
Lactose: 10.0g
Oxgall or oxbile solution: 200.0mL
0.1% brilliant green water solution: 13.3mL
Distilled water: 800mL
4.2.2 Preparation method
Dissolve peptone and lactose in about 500mL of distilled water, add 200mL of oxgall solution (dissolve 20.0g of dehydrated oxgall powder in 200mL of distilled water, and adjust the pH to 7.0~7.5), dilute to 975mL with distilled water, adjust the pH to 7.2±0.1, and then add 13.3mL of 0.1% brilliant green water solution, dilute to 1000mL with distilled water, filter the solution through cotton, and then dispense 10mL of the solution into each test tube which possesses small glass backward tubes. Sterilize for 15min in the autoclave set at 121℃.
4.3 Violet red bile agar (VRBA)
4.3.1 Composition
Peptone: 7.0g
Yeast extract: 3.0g
Lactose: 10.0g
Sodium chloride: 5.0g
Bile salt or No.3 bile salt: 1.5g
Neutral red: 0.03g
Crystal violet: 0.002g
Agar: 15g~18g
Distilled water: 1000mL
4.3.2 Preparation method
Dissolve all the above components in distilled water, stand for a few minutes, stir fully, and adjust the pH to 7.4±0.1. Boil for 2 min, melt and keep the temperature of the culture medium within the range of 45℃~50℃ and pour plate. Prepare it immediately before use, within 3 hours.
5. Note
In most countries, there are clear regulations on the methods for the detection and enumeration of coliforms, but the regulations may differ from country to country. This article mainly refers to the relevant inspection specifications of the People's Republic of China and is for reference only.
——————————————————————
Related Articles
1. Food Commercial Sterility Testing and Equipment
2. Equipment of Aerobic Plate Count
3. Equipment for testing and counting coliforms
4. Canned Preservation Principle (1) - General Knowledge
5. Canned Preservation Principle (2) - Cans Sizes
6. Canned Preservation Principle (3) - 3-piece Cans Resistance Welding
7. Canned Preservation Principle (4) - Cans Double Seam Seal
8. Canned Preservation Principle (5) - The Generation of Vacuum in the Can
9. Canned Preservation Principle (6) - Sterilization of Canned Food
10. Method of Drinking Water Automatic Treatment and Purification
—————————————————————— 
